
James P. Allison, PhD, director, immunotherapy platform, The University of Texas MD Anderson Cancer Center, discusses the future of immune checkpoint strategies.


James P. Allison, PhD, director, immunotherapy platform, The University of Texas MD Anderson Cancer Center, discusses the future of immune checkpoint strategies.

A new study has recommended priority targets for immunotherapy in epithelial ovarian cancer (EOC) by profiling the expression of certain tumor-associated antigens (TAA), called MAGE cancer-testis antigens (CTA), in a large panel of tumor samples.

The development of new immunotherapies for cancer treatment generated significant interest at the 2014 ASCO Annual Meeting, particularly checkpoint inhibitors targeting the PD-1 receptor and its ligand, PD-L1.
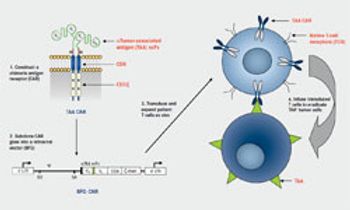
For nearly 2 decades, researchers have been exploring B-cell–specific antigens in hopes of developing a new anticancer target that could surpass the success of CD20-targeting agents.

Despite standard chemotherapy and the availability of targeted therapies such as bevacizumab, cetuximab, and tyrosine kinase inhibitors (TKIs) such as erlotinib, afatinib, and crizotinib, survival rates are far from optimal for patients with NSCLC.

The concept of cancer immunotherapy— using the patient’s immune system to target and eradicate cancer—has evolved over more than a century of research.

Amy P. Abernethy, MD, PhD, associate professor, School of Nursing, director, Duke Center for Learning Health Care, Duke University School of Medicine, discusses the results of a multisite randomized trial that examined continuing versus discontinuing statins in the setting of life-limiting illness.

Edward S. Kim, MD, chairman, Solid Tumor Oncology and Investigational Therapeutics, Levine Cancer Institute, Carolinas HealthCare System, discusses some of the challenges researchers face when it comes to biomarkers in lung cancer.

Lawrence Fong, MD, associate professor, Department of Medicine (Hematology/Oncology), University of California, San Francisco, discusses research to examine the immunobiology and biomarkers associated with improved clinical outcomes seen during treatment with an anti-CTLA-4 antibody.

The investigational CD19-targeted chimeric antigen receptor (CAR) therapy CTL019 has received a breakthrough therapy designation from the FDA as a potential treatment for pediatric and adult patients with relapsed/refractory acute lymphoblastic leukemia (ALL).

The PD-1 inhibitor nivolumab and the second-generation ALK inhibitor alectinib have each gained their first approvals as treatments for patients in Japan.

Naiyer A. Rizvi, MD, an associate attending physician, Memorial Sloan Kettering Cancer Center, discusses pembrolizumab (MK-3475) for the treatment of non-small cell lung cancer (NSCLC).
Frontline treatment with the anti-PD-1 agent nivolumab significantly extended overall survival (OS) when compared with dacarbazine for patients with metastatic or unresectable melanoma.

Jedd D. Wolchok, MD, PhD, discusses an updated analysis presented at the 2014 ASCO Annual Meeting that looked at pembrolizumab (MK-3475) for patients with melanoma.

Joseph Jurcic, MD, head, Hematologic Malignancies, Columbia University Medical Center, discusses using radioimmunotherapy as a treatment option for patients with AML.

The anti-PD-1 humanized antibody pembrolizumab (MK-3475) has robust antitumor activity as a first-line treatment for patients with advanced PD-L1-positive non–small cell lung cancer (NSCLC).

The FDA has approved the radioactive diagnostic imaging agent Lymphoseek injection to guide sentinel lymph node biopsy in patients with cancer of the head and neck.

Thomas E. Hutson, DO, PharmD, medical oncologist, Texas Oncology–Baylor Charles A. Sammons Cancer Center, discusses a phase I study of BPX-201 vaccine plus AP1903 for chemotherapy-naïve mCRPC.

David Spigel, MD, director, Lung Cancer Research Program, Sarah Cannon Research Institute, provides an update on research into MPDL3280A for the treatment of patients with lung cancer.

Ezra Cohen, MD, professor of medicine, UC San Diego Moores Cancer Center, discusses new data on immunotherapies for head and neck cancers.

James P. Allison, PhD, director, immunotherapy platform, The University of Texas MD Anderson Cancer Center, discusses the potentiation of immune checkpoint blockade cancer immunotherapy with oncolytic virus.

Phase I results presented in 2013 from a study combining ipilimumab with the anti-PD-1 antibody, nivolumab,1 showed a 40% objective response rate in 53 patients with advanced melanoma and a preliminary overall survival (OS) of 80%.

The intratumoral injection talimogene laherparepvec (T-VEC) demonstrated promise in combinations and utility as a monotherapy in certain subsets of patients with unresectable melanoma.

Pembrolizumab continues to deliver impressive results in patients with advanced melanoma—producing long-lasting responses and improved overall survival, regardless of whether patients have been previously treated with ipilimumab.

Two patients with metastatic cervical cancer achieved durable complete responses that have so far lasted from 15 to 22 months through an adoptive T-cell therapy (ACT) targeting the human papillomavirus (HPV).