
Clinicians will soon face challenging decisions on which immunotherapies to prescribe their patients, and in what sequence or combination.

Clinicians will soon face challenging decisions on which immunotherapies to prescribe their patients, and in what sequence or combination.

Multiple trials are ongoing in NSCLC with immunotherapy agents. As with most cancers, however, there is also a need to identify which types of patients with NSCLC might benefit the most from these new therapies.

Vassiliki Papadimitrakopoulou, MD, discusses adverse events associated with immunotherapies for the treatment of lung cancer.

In malignancies with limited response rates to existing radiation and/or chemotherapy, immunotherapy is being investigated as a potential adjunct, with promising results, particularly in the treatment of malignant mesothelioma.

Barbara Burtness, MD, discusses potential immunotherapy agents that may assist in the treatment of head and neck cancers.
It is estimated that 1 in 63 individuals in the United States will develop renal cell carcinoma (RCC), making it among the most common cancers in the country.

William K. Oh, MD, discusses immunotherapies for the treatment of patients with prostate cancer.
The FDA has granted the chimeric antigen receptor (CAR) T cell therapy JCAR015 a breakthrough therapy designation as a treatment for patients with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL).
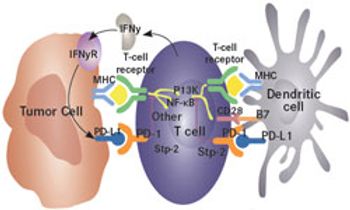
Checkpoint inhibition has demonstrated efficacy for the treatment of melanoma in several clinical trials. However, biomarkers to predict safety and efficacy of immunotherapies in individual melanoma patients are lacking.

Ongoing clinical trials are evaluating a new immunotherapeutic approach for the treatment of malignant melanoma–the combination of an oncolytic virus with checkpoint inhibition.

Scientists are beginning to find that combination therapy improves outcomes for patients, particularly with ipilimumab-nivolumab combination therapy.
As its CAR T cell and high-affinity TCR products continue to advance in clinical trials, Juno Therapeutics, Inc, filed a registration statement for an initial public offering (IPO) of its common stock on November 17.

Pembrolizumab (Keytruda) significantly improved progression-free survival (PFS) by over 43% compared with chemotherapy as a treatment for patients with metastatic melanoma who were refractory to ipilimumab (Yervoy).

Frontline treatment with nivolumab (Opdivo) significantly extended overall survival (OS) and progression-free survival (PFS) when compared with dacarbazine in patients with untreated BRAF wild-type advanced melanoma.

Several novel approaches to the delivery of therapeutics for advanced melanoma may improve treatment efficacy and patient survival.
A plenary session held November 15 at the Society of Neuro-Oncology’s (SNO) 2014 Annual Meeting in Miami Beach focused on immunotherapy’s promise as well as its challenges as a treatment for patients with brain cancer.

According to data from a phase I study, the oncolytic virus Delta-24-RGD can infect, replicate, and kill glioma cells in patients.

David Reardon, MD, clinical director, Center for Neuro-Oncology, Dana-Farber Cancer Institute, president, Society for Neuro-Oncology, describes the mechanism of action of rindopepimut for recurrent glioblastoma.
The vaccine rindopepimut appears to benefit patients with epidermal growth factor receptor variant III mutation (EGFRvIII) in glioblastoma with regard to progression-free survival (PFS) and overall survival (OS).

The vaccine rindopepimut (CDX110) in combination with bevacizumab induced tumor regression in a subset of patients with recurrent glioblastoma.

Recent news stories profiling a cancer patient whose last hope rests on treatment by injections of the virus that causes AIDS may have created some misconceptions regarding a new cancer immunotherapy.

Combination therapies with agents such as indoleamine 2,3-dioxygenase (IDO) inhibitors may have the potential to synergize with immunotherapeutic approaches to improve immune function against tumor cells.

Peter Hammerman, MD, PhD, assistant professor of medicine, Harvard Medical School, Dana-Farber Cancer Institute, discusses the future treatment of squamous cell lung cancer.

Abraham Chachoua, MD, discusses some challenges of treating patients with lung cancer.

Dendreon has filed for chapter 11 bankruptcy, the company announced Nov. 10. Dendreon is the maker of the first FDA-approved immunotherapy for advanced prostate cancer, sipuleucel-T (Provenge).