
International Symposium on Melanoma May Set Foundations for Clinical Decision Making
Omid Hamid, MD, discusses the upcoming Annual International Symposium on Melanoma and Other Cutaneous Malignancies.

Omid Hamid, MD, discusses the upcoming Annual International Symposium on Melanoma and Other Cutaneous Malignancies.

Saad Usmani, MD, discusses newly approved treatments, as well as these combination therapies currently in clinical trials.

With numerous newly approved drugs for patients with early-stage multiple myeloma reaching the clinics, understanding what drugs, combinations, and sequences to use has become an important area of research. Several recent studies have demonstrated increased efficacy with nominally increased adverse events in these triplet arms.

The phase III PILLAR trial studying the novel immunotherapy, algenpantucel-L combined with chemotherapy in patients with locally advanced, unresectable pancreatic cancer, has completed enrollment with over 300 patients.
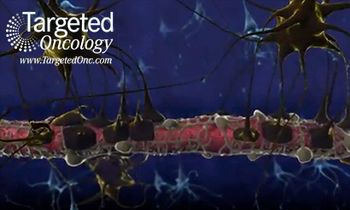
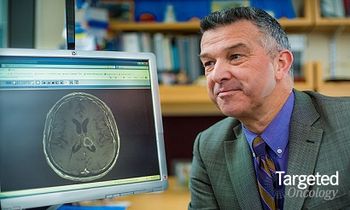
A team of scientists have conducted preclinical trials of a novel drug nanocarrier, 3HM, that may offer a solution to breaking the blood-brain barrier in the treatment of brain cancers like glioblastoma multiforme.

Select medical centers nationwide are now offering blue-light cystoscopy imaging to better detect papillary cancer of the bladder.

Study data involving NY-ESO-1specific T-cell receptor transgenic lymphocytes with an NY-ESO-1 peptide-pulsed DC vaccination in patients with advanced sarcomas and melanoma with or without ipilimumab were detailed during a presentation at the 2015 CTOS Annual Meeting.

TRC105, an anti-endoglin antibody, demonstrated complementary antitumor effects with pazopanib, a VEGF inhibitor in the treatment of patients with soft tissue sarcomas.

The 2015 NANETS Annual Symposium begins October 15 and the theme focuses on a team perspective. Kari Brendtro, founder of NANETS, recently spoke with Targeted Oncology about some of this year's upcoming presentations.
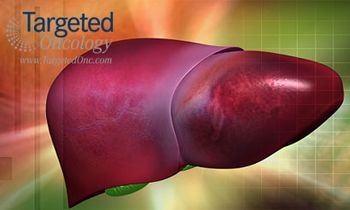
Researchers from United States and Ireland have formed a partnership to advance nanoparticle-based delivery systems that would not only drive sustained-release of cytotoxic agents, but could improve penetration of these treatments within the tumor and allow them to continue working for days or weeks.

An experimental immunotherapy for human papillomavirus-, or HPV-, related throat cancers, which is driven by the Listeria bacteria (that wreaks havoc when ingested), may now move forward.
Abstracts highlighting the latest clinical data on lenvatinib are expected at the 2015 European Cancer Congress in Vienna, Austria.
Predictive data from two major financial research and consulting firms, UBS and GlobalData, have supported atezolizumab [MPDL3280A] as a major player in the immunotherapy race for bladder cancer.

A study including the largest reported US cohort of patients under age 35 with colorectal cancer (CRC) has discovered that one-third of these patients suffer from hereditary forms of the disease.
An independent data safety and monitoring board has recommended continuation of the phase III ACT IV clinical trial of rindopepimut (Rintega) in patients with newly diagnosed glioblastoma multiforme.

Abiraterone acetate in combination with low-dose prednisone (5 mg) significantly lowered PSA levels with a consistent toxicity profile in men with high-risk nonmetastatic castration-resistant prostate cancer.

Joyce A. O’Shaughnessy, MD, chair, breast cancer service at Baylor-Sammons Cancer Center provided Targeted Oncology with a sneak peek into some of the recent advances in breast cancer that will be discussed at the 14th Annual International Congress on the Future of Breast Cancer®

Almost half of patients with cancer who receive genetic testing may be given information that leads to unsuitable targeted treatments, according to researchers from Johns Hopkins Kimmel Cancer Center.

Although treatments and cure rates have increased significantly over the past 60 years for patients with HL, it is crucial that practitioners stay up-to-date on research that can affect outcomes for their patients with this uncommon form of cancer.

The FDA has granted a breakthrough therapy designation to pembrolizumab for the treatment of patients with NSCLC who are EGFR mutation- or ALK rearrangement-negative and whose disease has progressed on or following platinum-based chemotherapy.

In 2001, the FDA approved Gleevec (imatinib mesylate), a therapy for CML that was quickly labeled a magic bullet, and shortly thereafter, the International Congress on Targeted Therapies in Cancer® was assembled to discuss what these types of therapies would mean for the cancer treatment of the future.

One of the first studies to prospectively examine women’s breast surgery preferences has revealed that newly diagnosed women with breast cancer who decide to undergo contralateral prophylactic mastectomy (CPM), may be making this decision as result of high anxiety and fear of recurrence.

New and evolving strategies for clinical trial design are under way, a point that was made abundantly clear April 25th at the NCCN Policy Summit on Designing Clinical Trials in the Era of Multiple Biomarkers and Targeted Therapies.

Phase I results presented in 2013 from a study combining ipilimumab with the anti-PD-1 antibody, nivolumab,1 showed a 40% objective response rate in 53 patients with advanced melanoma and a preliminary overall survival (OS) of 80%.

A joint analysis of two phase III trials involving a total of 4690 premenopausal women with HR+ breast cancer demonstrated that adjuvant use of exemestane reduced relative risk of developing subsequent invasive cancer by 28% compared with tamoxifen.

MPDL3280A received the first Breakthrough Therapy Designation from the FDA for bladder cancer, according to information released May 31, 2014 at the 50th Annual Meeting of ASCO.

Patients starting palliative care for advanced cancer are often already on repeat prescriptions of statins to lower cholesterol and reduce risk of a heart attack or stroke, and many of these patients continue on statin therapy until death.
The FDA Department of Orphan Products Development has granted orphan drug designation to demcizumab (anti-DLL4, OMP-21M18), which is currently in a phase Ib clinical trial in combination with Abraxane® (nab-paclitaxel) and gemcitabine.

Increasing efficacy with cancer vaccines, oncolytic viruses, and checkpoint inhibitors in clinical trials led to immunotherapy, as a whole, being deemed Breakthrough of the Year by Science magazine in 2013.

At the 10th International Meeting of the Society for Melanoma Research, researchers provided insight into combination treatments and immunotherapies for the treatment of melanoma.
Published: May 6th 2014 | Updated: April 17th 2020

Published: October 27th 2014 | Updated: April 17th 2020

Published: April 15th 2015 | Updated: April 17th 2020

Published: May 19th 2015 | Updated: April 17th 2020
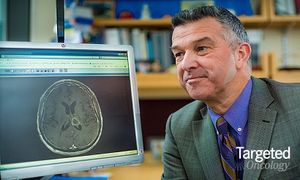
Published: June 30th 2015 | Updated: April 17th 2020

Published: July 24th 2015 | Updated: April 17th 2020