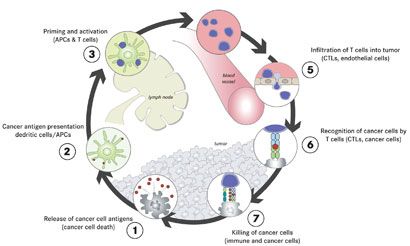
IMMUNOTHERAPY
Latest News
Latest Videos
More News

Omid Hamid, MD, discusses sequencing and combining targeted therapies in the treatment of melanoma.
An immune checkpoint inhibitor combined with active immunotherapy has a potential positive effect on overall survival (OS) in the treatment of metastatic castration-resistant prostate cancer (mCRPC).
In an analysis of adverse events following treatment of patients with advanced melanoma with ipilimumab and nivolumab, combination therapy was associated with a 22% incidence of either thyroiditis or hypothyroidism and a 9% incidence of hypophysitis.

MPDL3280A, an investigational antibody that targets programmed death-ligand 1 (PD-L1), in combination with bevacizumab had strong antitumor activity and induced responses in 4 of 10 patients with mRCC.

The FDA has approved nivolumab (Opdivo) for the treatment of patients with advanced non-small cell lung cancer (NSCLC). The approval comes 3 months ahead of the FDA’s scheduled decision date.

The FDA has accepted a supplemental Biologics License Application (sBLA) for ipilimumab (Yervoy) as an adjuvant treatment of patients with stage III melanoma at high risk of recurrence following complete resection.
Nivolumab (Opdivo) has been granted a priority review for use in patients with previously treated, advanced, squamous non–small cell lung cancer (NSCLC).

Jae Park, MD, assistant attending physician, Memorial Sloan Kettering Cancer Center, discusses toxicities associated with CAR-modified T cell therapy.
An orphan drug designation has been granted to Reolysin (wild-type reovirus) for the treatment of patients with ovarian cancer.

The FDA has scheduled a hearing to discuss the biologics license application (BLA) for the immunotherapy talimogene laherparepvec (T-VEC) as a treatment for patients with metastatic melanoma.

Representatives Diana DeGette (D, Colorado) and Fred Upton (R, Michigan) recently released a "discussion draft" of the 21st Century Cures Act.

Kei Muro, MD, discusses phase II and III trials which examine pembrolizumab as a treatment for patients with advanced gastric cancer.

The anti–PD-L1 agent MPDL3280A has received a breakthrough therapy designation from the FDA as a potential treatment for patients with PD-L1–positive NSCLC who have progressed on platinum-based chemotherapy and a EGFR or ALK inhibitor.
Metastatic disease accounts for the vast majority of cancer-related deaths. Ensuring a definitive diagnosis and the most effective treatment in a timely fashion is essential for extending life expectancy.

Pembrolizumab showed promising antitumor activity and a manageable toxicity profile in patients with metastatic gastric cancer, according to updated findings from the KEYNOTE-012 study.

The start of 2015 brought news from Novartis that it had signed an agreement with Intellia Therapeutics and Caribou Biosciences to license its proprietary CRISPR/Cas9 gene editing platform to develop novel treatments for chronic genetic-based diseases.

Nivolumab (Opdivo) improved survival compared with docetaxel in patients with pretreated squamous cell non–small cell lung cancer (NSCLC) in the phase III CheckMate-017 trial, according to Bristol-Myers Squibb (BMS), which manufactures the drug.

One of the leading non-clinical attributes that affect how oncologists decide which therapeutic agent to prescribe over another is patient affordability.

Amgen and Kite Pharma have announced that they will collaborate on the development of novel CAR T-cell immunotherapies, with Amgen providing cancer targets and Kite offering its engineered autologous cell therapy platform.

The FDA approved nivolumab for patients with advanced melanoma in December 2014, joining 6 other melanoma treatments approved in the past 3 years, including monoclonal antibodies pembrolizumab and ipilimumab.

The FDA has approved nivolumab (Opdivo) for patients with unresectable or metastatic melanoma following treatment with ipilimumab or a BRAF inhibitor, based on data from the phase III CheckMate-037 trial.

Edith A. Perez, MD, the deputy director at large for the Mayo Clinic Cancer Center, comments on immune checkpoint inhibition for the treatment of breast cancer.

Sara Hurvitz, MD, medical oncologist, UCLA Medical Center, discusses the results of a study looking at pembrolizumab (Keytruda) in patients with triple-negative breast cancer (TNBC).

Jae Park, MD, assistant attending physician, Memorial Sloan Kettering Cancer Center, discusses CAR T-cell therapy for the treatment of acute lymphoblastic leukemia.

The anti-CD19 chimeric antigen receptor (CAR)-modified T-cell therapy CTL019 demonstrated a 92% complete response (CR) rate in pediatric patients with relapsed/refractory acute lymphoblastic leukemia (ALL).