
More than 50% of melanoma involves mutation in the BRAF protein, which is part of the mitogen-activated protein kinase (MAPK) signal transduction pathway.

More than 50% of melanoma involves mutation in the BRAF protein, which is part of the mitogen-activated protein kinase (MAPK) signal transduction pathway.

The first combination therapy for advanced melanoma received approval from the Food and Drug Administration (FDA) January 2014.

Jedd D. Wolchok, MD, PhD, chief, Melanoma and Immunotherapeutics Service, Lloyd J. Old Chair for Clinical Investigation, Memorial Sloan Kettering Cancer Center, explains immune checkpoint blockade.

Melanoma experts and researchers have gained ground in the development of novel and effective immunotherapies as well as targeted agents for those patients with metastatic melanoma who harbor specific tumor mutations.

Keith T. Flaherty, MD, discusses the latest and forthcoming research regarding MEK/BRAF combination therapies.

Ryan J. Sullivan, MD, medical oncologist, Massachusetts General Hospital, gives an overview of new and emerging therapies for the treatment of metastatic melanoma.
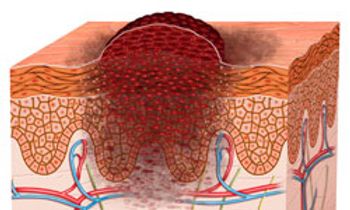
The American Cancer Society estimates that more than 75,000 new cases of melanoma will be diagnosed in 2014, and that nearly 10,000 people will die of melanoma this year.

Targeted therapy for patients with activating BRAF mutations has demonstrated the potential for personalized medicine in patients with metastatic melanoma. Initial study results were promising and dramatic.

PD-L1 levels adequately predict response and clinical outcomes for PD-1 inhibitor MK-3475 in patients with non-small cell lung cancer (NSCLC) and melanoma.

The oncolytic immunotherapeutic vaccine talimogene laherparepvec (T-VEC) promoted tumor shrinkage in 64% of patients with advanced melanoma, including a marked reduction in the size of uninjected metastatic lesions.

Nivolumab, a PD-1-specific antibody, has shown to produce long-term remissions with limited toxicity in patients with advanced melanoma, according to results from one of the longest follow ups to examine the drug.

While the concept of cancer-specific immunotherapy is not new, it recently has been proven feasible as a rational treatment for patients with some of the most challenging and difficult malignancies.

Harriet Kluger, MD, associate professor of medicine (medical oncology), associate director, Hematology/Oncology Fellowship Program, Yale Cancer Center, explains how immunotherapies are changing the treatment of melanoma.

Until recently, the cornerstone of therapy for metastatic melanoma had been chemotherapy with dacarbazine (DTIC) and immunotherapy with high-dose interleukin-2 (HD IL-2) or interferon- (IFN- ).

Antoni Ribas, MD, PhD, professor of medicine, Jonsson Comprehensive Cancer Center, University of California, Los Angeles, discusses the excitement surrounding immunotherapies for the treatment of patients with melanoma.

Georgina Long, BSc, PhD, MBBS, FRACP, medical oncologist, translational researcher, Melanoma Institute Australia, The University of Sydney, highlights targeted therapies in development for melanoma.

Skin cancer is the most common form of malignancy in the United States, and melanoma represents the most deadly subset.

Updates on dabrafenib, trametinib, lambrolizumab, and more.
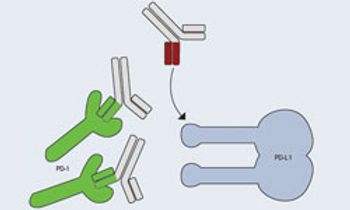
There are currently numerous experimental therapeutic options in various phases of clinical development that may hold promise for patients with advanced melanoma.

Merck announced the signing of three separate clinical collaboration agreements to evaluate the potential of MK-3475 across multiple tumor types. The agreements were signed through subsidiaries with Amgen Inc., Incyte Corporation, and Pfizer Inc.

Harriet Kluger, MD, associate professor of medicine (medical oncology), associate director, Hematology/Oncology Fellowship Program, Yale Cancer Center, comments on the changing landscape of immunotherapies for the treatment of melanoma.

The combination of the MEK inhibitor trametinib (Mekinist) and the BRAF inhibitor dabrafenib (Tafinlar) has received an accelerated approval from the FDA as a treatment for patients with unresectable or metastatic melanoma who harbor a BRAF V600E or V600K mutation.

The discovery of two unique molecular targets known to speed skin-cancer growth has researchers excited that they might soon understand and develop genetic therapies geared toward some of medicine’s most untreatable melanomas.

Most cases of BCC are curable with such approaches as surgery and radiation. In some cases, however, BCC can progress to a point of local invasion for which surgery and radiation therapy are not indicated.

Although the diagnosis of late-stage melanoma is still associated with a poor prognosis, an encouraging number of new therapies have been developed during the last 3 years.