
Since the FDA approval of ipilimumab, there has been considerable interest in the potential for other immune system checkpoints, such as PD-L1 and its receptor, PD-1, to serve as therapeutic targets in metastatic melanoma.
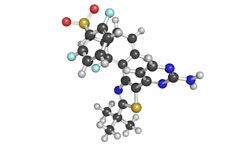

Since the FDA approval of ipilimumab, there has been considerable interest in the potential for other immune system checkpoints, such as PD-L1 and its receptor, PD-1, to serve as therapeutic targets in metastatic melanoma.

Bristol-Myers Squibb has filed a lawsuit over Merck’s newly approved immunotherapy drug pembrolizumab, contending that the much-heralded PD-1 inhibitor will infringe upon patents that Bristol-Myers holds on the groundbreaking technology.

The FDA has approved pembrolizumab (Keytruda) as a treatment for patients with advanced or unresectable melanoma following progression on prior therapies.

Recent advances in immunotherapy and in agents that target specific genetic mutations in the mitogen-activated protein kinase (MAPK) pathway have led to dramatic improvements in outcomes for patients with advanced cutaneous melanoma.

Igor Puzanov, MD, medical oncologist, Vanderbilt-Ingram Cancer Center, discusses the rationale behind a phase Ib study to evaluate the efficacy and safety of T-VEC and ipilimumab in previously untreated, unresected stage IIIB-IV melanoma
Risk factors for contracting melanoma are complex and can include environmental exposure and an individual’s genetic susceptibility, but people at highest risk are those with a personal or family history of melanoma and those with large, atypical, and/or multiple (more than 50) nevi.

Mario Sznol, MD, professor, Internal Medicine, Yale Cancer Center, discusses a phase I trial that examined the combination of nivolumab and ipilimumab for the treatment of advanced melanoma. The data presented at the 2014 ASCO Annual Meeting was an updated survival and clinical activity analysis in initially enrolled cohorts and activity by BRAF mutation status.

A phase III study comparing trametinib (Mekinist) plus dabrafenib (Tafinlar) with single-agent vemurafenib (Zelboraf) has been stopped early following a positive interim analysis.
The combination of the BRAF inhibitor vemurafenib and the MEK inhibitor cobimetinib significantly improved progression-free survival (PFS) compared with vemurafenib alone for patients with untreated BRAFV600-mutated advanced melanoma.

The PD-1 inhibitor nivolumab and the second-generation ALK inhibitor alectinib have each gained their first approvals as treatments for patients in Japan.

Loren Clarke, MD, vice president of Medical Affairs of Dermatology at Myriad Genetics, discusses the company’s new myPath test for melanoma
Frontline treatment with the anti-PD-1 agent nivolumab significantly extended overall survival (OS) when compared with dacarbazine for patients with metastatic or unresectable melanoma.

Jedd D. Wolchok, MD, PhD, discusses an updated analysis presented at the 2014 ASCO Annual Meeting that looked at pembrolizumab (MK-3475) for patients with melanoma.

The FDA has approved the radioactive diagnostic imaging agent Lymphoseek injection to guide sentinel lymph node biopsy in patients with cancer of the head and neck.

Gregory A. Daniels, MD, PhD, Associate Clinical Professor of Medicine, Division of Hematology-Oncology, University of California, San Diego, discusses the findings of the 2007-2012 PROCLAIM national registry

Phase I results presented in 2013 from a study combining ipilimumab with the anti-PD-1 antibody, nivolumab,1 showed a 40% objective response rate in 53 patients with advanced melanoma and a preliminary overall survival (OS) of 80%.

The intratumoral injection talimogene laherparepvec (T-VEC) demonstrated promise in combinations and utility as a monotherapy in certain subsets of patients with unresectable melanoma.

Pembrolizumab continues to deliver impressive results in patients with advanced melanoma—producing long-lasting responses and improved overall survival, regardless of whether patients have been previously treated with ipilimumab.

The immunotherapy drug ipilimumab (Yervoy) reduced the relative risk of cancer recurrence in the adjuvant setting by 25% compared to placebo for patients with high-risk, lymph-node positive (stage III) melanoma.

Despite improved efficacy with new therapies, both as monotherapy or in combinations, they provide new challenges to nurses in managing side effects and adjusting treatment.

Jedd D. Wolchok, MD, PhD, from Memorial Sloan Kettering Cancer Center, discusses ongoing research regarding combinations of immunotherapies.

The combination of vemurafenib with the investigational MEK inhibitor cobimetinib demonstrated a 13.7-month median PFS and an ORR of 87% in treatment-naïve patients with BRAFV600 mutation-positive metastatic melanoma.

Michael A. Postow, MD, attending physician, Melanoma and Immunotherapeutics Service, Memorial Sloan Kettering Cancer Center, discusses using PD-L1 as a biomarker of response in melanoma.

The FDA has assigned a priority review designation to the PD-1 inhibitor pembrolizumab (MK-3475) as a treatment for patients with unresectable or metastatic melanoma following progression on ipilimumab.

Many patients with melanoma are diagnosed with unresectable stage III or IV disease that requires systemic treatment. Treatment of melanoma depends largely on the stage at diagnosis.