
Samer A. Al’Hadidi, MD, discusses the follow-up data for ciltacabtagene autoleucel in patients with multiple myeloma.

Samer A. Al’Hadidi, MD, discusses the follow-up data for ciltacabtagene autoleucel in patients with multiple myeloma.

Luis E. Raez, MD, discusses the current liquid biopsy options for patients with lung cancer through assessing circulating tumor DNA.

James J. Harding, MD, provides a high-level overview of systemic therapy for untreated unresectable hepatocellular carcinoma (uHCC) based on NCCN Guidelines, and discusses the evolving treatment landscape, including the rationale for combination immunotherapy (IO) and how additional IO combinations may address unmet needs like depth of response and resistance.
Petros Grivas, MD, PhD, discusses the key trials of bladder cancer using immunotherapy he highlighted during a Case-Based Roundtable event.

Eunice S. Wang, MD, discusses research behind optimal therapy for acute myeloid leukemia secondary to other malignancies, particularly in patients with prior myelodysplastic syndrome.

Alexa Simon Meara, MD, discusses the importance of including a rheumatology approach to immunotherapy treatment for cancer.

Panelists discuss how recent therapeutic advances, including novel drug combinations and personalized treatment approaches, are reshaping the standard of care for patients with newly diagnosed multiple myeloma (NDMM) while emphasizing the importance of risk stratification and early intervention strategies.
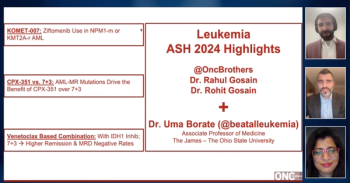
With the Oncology Brothers, Uma Borate, MD, discusses how the combination of venetoclax with an IDH1 inhibitor and 7+3 chemotherapy leads to higher remission rates and MRD negativity in IDH1-mutated AML, offering a promising treatment option for these patients.

With the Oncology Brothers, Uma Borate, MD, discusses how CPX-351 demonstrates superior efficacy over 7+3 chemotherapy in treating acute myeloid leukemia with mutations in measurable residual disease (AML-MR) by specifically targeting the genetic mutations driving the disease, leading to improved patient outcomes.

With the Oncology Brothers, Uma Borate, MD, discusses how ziftomenib is showing promising results as a targeted therapy for NPM1-mutated and KMT2A-rearranged acute myeloid leukemia (AML) in the ongoing KOMET-007 trial, offering potential new treatment options for this challenging patient population.

Hope S. Rugo, MD, FASCO, highlights key data from the ELEVATE study, presented at SABCS 2024.

Panelists discuss how frontline luspatercept is emerging as an effective treatment for anemia in lower-risk myelodysplastic syndrome (LR-MDS), demonstrating significant improvements in hemoglobin levels and transfusion independence, as supported by clinical trial data.

Panelists discuss how the NCCN Guidelines define lower-risk myelodysplastic syndrome (LR-MDS) based on clinical hallmarks such as anemia and cytopenias, with treatment decisions guided by prognostic scoring systems (IPSS-R, IPSS-M), and how real-world data supports the evolving first-line treatment landscape for LR-MDS, including the use of therapies such as luspatercept.

Hope S. Rugo, MD, FASCO, discusses how the phase 3 ELEVATE study evaluates the combination of elacestrant with abemaciclib in patients with ER+/HER2– advanced or MBC who have progressed on prior endocrine therapy.

Joe Leach, MD, discusses how comprehensive biomarker testing has impacted treatment selection for patients with small cell lung cancer.

Paolo Ghia, MD, PhD, provides an overview of the CAPTIVATE trial, uncovering what investigators sought to evaluate.

Evan Y. Yu, MD, discusses how the goals of therapy for a patient with newly- diagnosed metastatic hormone-sensitive prostate cancer are to control disease progression, extend survival, maintain quality of life, and delay the transition to castration-resistant prostate cancer.

Evan Y. Yu, MD, introduces case 1, a 68-year-old patient with newly diagnosed metastatic hormone-sensitive prostate cancer (mHSPC), highlighting the clinical presentation, diagnostic workup, and treatment considerations for high-volume metastatic disease.

Jaime Merchan, MD, discusses the efficacy results observed in the LITESPARK-013 trial of belzutifan in patients with advanced clear cell renal cell carcinoma.

Josep M. Llovet, MD, PhD, describes the phase 3 LEAP-012 clinical study, including its methods, design, and enrollment criteria.

Anita D'Souza, MD, discusses an abstract looking at the combination of teclistamab-cqyv, daratumumab, and pomalidomide in 2 phase studies for patients with multiple myeloma.

Hope S. Rugo, MD, FASCO, discusses how the combination of elacestrant and abemaciclib compares with other treatment options for patients with advanced breast cancer, whether estrogen receptor-positive or HER2-negative.

Panelist discusses how talquetamab’s step-up dosing strategy aims to mitigate cytokine release syndrome, a common immunologic toxicity. The reported grade 1 CRS with altered taste and dry mouth represents a mild manifestation compared with the MonumenTAL-1 trial, where the initial SUD schedule showed varying CRS rates. Real-world evidence has largely validated trial findings, though alternative SUD approaches may offer different risk-benefit profiles. The optimal SUD strategy continues to evolve as clinical experience expands, balancing efficacy with tolerability.

Panelist discusses how the MonumenTAL-1 study led to the approval of talquetamab in patients with 4 or more prior lines of therapy, and the initial response rate was 70%. There were also several toxicities associated with MonumenTAL-1, including oral, nail, and skin toxicities.

Virginia G. Kaklamani, MD, DSc, discusses how effective elacestrant (Orserdu) is in CDK4/6 inhibitor-naive estrogen receptor-positive/HER2-negative metastatic breast cancer treatment.

Abdulraheem Yacoub, MD, discusses option for patients with myelodysplastic syndromes and red blood cell transfusion dependence.

Anita D'Souza, MD, discusses some of the potentially practice-changing data that were presented at the 2024 ASH Annual Meeting.

Aditya Bardia, MD, MPH, FASCO, discusses how fam-trastuzumab deruxtecan-nxki compares with physician’s choice of chemotherapy in estrogen receptor-positive/HER2-low breast cancer.

A panelist discusses how combining elacestrant with targeted therapies like CDK4/6 inhibitors or PI3K inhibitors could potentially enhance its efficacy and overcome resistance mechanisms in ER+/HER2– MBC treatment.

Hope S. Rugo, MD, FASCO, discusses how elacestrant, an oral selective estrogen receptor degrader, demonstrated superior progression-free survival compared with standard endocrine therapy in patients with ER+/HER2– metastatic breast cancer (MBC) who had previously received CDK4/6 inhibitors in the phase 3 EMERALD trial.