
MPNs
Latest News
Latest Videos

More News

A rolling submission of a New Drug Application has been initiated for pacritinib, seeking FDA approval for the drug as treatment of patients with myelofibrosis with severe thrombocytopenia.

During the National Comprehensive Cancer Network 2020 Virtual Congress: Hematologic Malignancies, Aaron Gerds, MD, MS, reviewed the current treatment landscape for patients with myelofibrosis and what’s to come for the treatment of this patient population as clinical trials continue to advance the field.

A New Drug Application for pacritinib is planned to be submitted for potential accelerated approval from the FDA for the treatment of patients with myelofibrosis and severe thrombocytopenia.

Raajit K. Rampal, MD, PhD, discussed the role of genomics in the treatment landscape of myelofibrosis and the remaining challenges that need to be addressed in order to use this information more effectively to treat patients and improve outcomes.

In an interview with Targeted Oncology, Naveen Pemmaraju, MD, discussed recent advances for the treatment of patients with MPNs and shared his thoughts on recent hematologic meetings and the growing role of social media during the COVID-19 pandemic.

Ruben Mesa, MD, shares highlights from his presentation on the role of JAK inhibitors in myelofibrosis during the first annual Texas Virtual MPN Workshop and Meeting.

In an interview with Targeted Oncology, Prithviraj Bose, MD, discussed the disease outcomes for patients with essential thrombocythemia, as well as the unmet needs and therapeutic strategies available in this space.
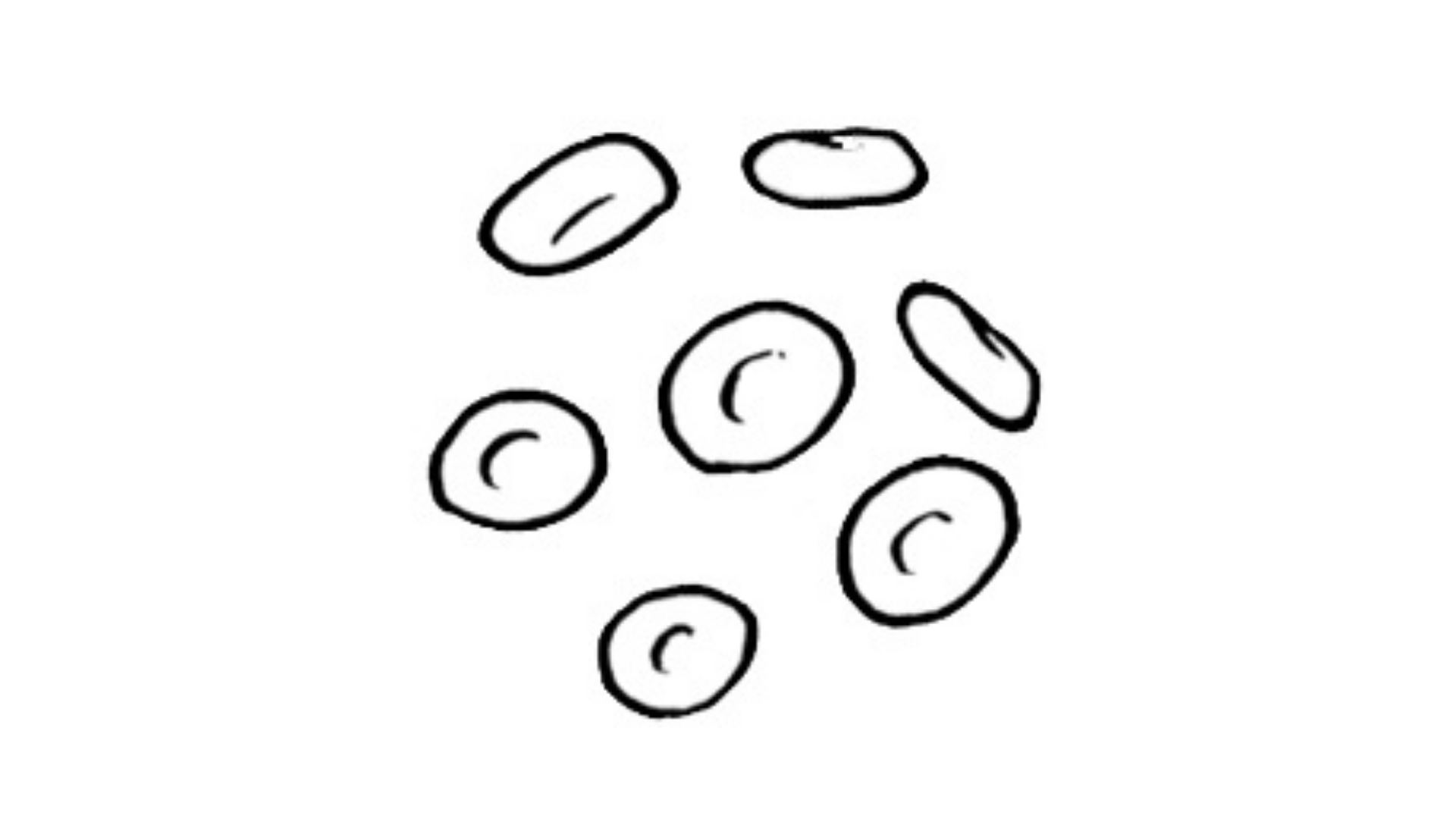
Prognostic factors in patients with myelofibrosis treated with a myeloablative busulfan/fludarabine conditioning regimen prior to allogeneic stem cell transplantation in a single-institution analysis showed that a time from diagnosis to transplant of more than 12 months was associated with poorer overall survival and displayed a trend toward higher non-relapse mortality.

JAK inhibitors continue to make a splash in the treatment landscape of MPNs and are be evaluated in multiple clinical trials, and the advancement of these agents should be recognized in honor of MPN Awareness Day.

Naveen Pemmaraju, MD, discusses the need to move beyond JAK inhibition when treating patients with myeloproliferative neoplasms.

Experts in myeloproliferative neoplasms find janus kinase inhibitors to be particularly important to the armamentarium for the treatment of myelofibrosis. With only 2 FDA-approved agents, fedratinib and ruxolitinib, and the inevitability that not all patients will derive benefit, and some will develop resistance, the option of moving beyond JAK inhibition is widely discussed.

Patients with myelofibrosis have complicated pathology and multiple pathways, creating the opportunity to use multiple targeted agents for treatment, but also leading to greater potential for resistance to monotherapy, according to Lucia Masarova, MD.

Standard treatment for accelerated or blast phase myeloproliferative neoplasms consists of hypomethylating agents or intensive induction chemotherapy and transplant. However, newer studies have suggested that accelerated or blast phase MPNs, such as acute myeloid leukemia, can be treated with molecularly driven targeted therapies.

In an interview with Targeted Oncology, Ruben Mesa, director, MD, discussed the role of JAK inhibitors in early myelofibrosis and other promising treatment options coming down the pipeline.

During the 1st Annual Texas MPM Workshop, Aaron Gerds, MD, MS presented on the ruxolitinib in the present and future, as well as the possibilities for moving beyond the JAK-STAT pathway.

Patients considered to have early myelofibrosis are a heterogeneous group for whom disease risk, best treatment strategies, and the probability of mortality are best determined individually by looking at patient’s clinical characteristics and molecular markers together.

Srdan Verstovsek, MD, PhD, discusses the data seen in the phase 2 trial of CPI-0610 with or without ruxolitinib in patients with myelofibrosis.

Ruben Mesa, MD, reviewed a case of a 68-year-old woman with a myeloproliferative neoplasm, during a virtual Case Based Peer Perspectives event.

Ruxolitinib induced clinically meaningful reductions in both spleen size and symptoms for patients with myelofibrosis, including those with low platelet counts.

In an interview with Targeted Oncology, Srdan Verstovsek, MD, PhD, discussed the data from the MANIFEST trial, which evaluated the use of CPI-0610 as treatment of patients with myelofibrosis.

Srdan Verstovsek, MD, PhD, discusses the potential role of CPI-0610 as treatment of patients with myelofibrosis.

The approval of ruxolitinib may have generated an increased awareness around myelofibrosis, ultimately improving the overall management of this patient population.

In an interview with Targeted Oncology, Claire Harrison, MD, FRCP, FRCPath, discussed results from the combination of navitoclax and ruxolitinib, as explored in a phase 2 study.

During the Virtual 25th Congress of the European Hematology Association (EHA), a group of physicians described their experiences and recommendations for managing patients with myeloid malignancies during the COVID-19 pandemic and after.

These data suggest that screening for pulmonary hypertension is warranted in patients with a Philadelphia chromosome-negative myeloproliferative neoplasm, both at the time of diagnosis and during follow-up.



























