
In an interview with Targeted Oncology™, Kamran A. Ahmed, MD, discussed the novel treatment strategy of combining radiotherapy with intrathecal trastuzumab/pertuzumab as well as and the rationale behind the combination.

In an interview with Targeted Oncology™, Kamran A. Ahmed, MD, discussed the novel treatment strategy of combining radiotherapy with intrathecal trastuzumab/pertuzumab as well as and the rationale behind the combination.

In an interview with Targeted Oncology™, Shannon N. Westin, MD, MPH, FACOG, discussed the DUO-E trial, its design, end points, and the need for novel endometrial cancer therapies.

A warning from the FDA highlights that an increased rate of death has been observed in patients with multiple myeloma who are undergoing treatment with the FDA-approved agent melphalan flufenamide in combination with dexamethasone in the phase 2 OCEAN clinical trial.

Selinexor has been administered in combination with ruxolitinib to the first patient with treatment-naïve myelofibrosis as part of a phase 1/2 clinical trial.

The first patient with an advanced solid tumor has been dosed with the first-in-class anti-PVRIG antibody, COM701, in combination with nivolumab and the anti-TIGIT antibody, BMS-986207, in a phase 1/2 clinical trial.

A phase 3 study has been initiated in order to evaluate the efficacy of the oral MDM2 inhibitor milademetan for the treatment of de-differentiated liposarcoma.

The first patient has been dosed in the phase 1/2a clinical trial, designed to evaluate LAVA-051- a gamma-delta bispecific gamma-delta T cell engager that activates Vγ9Vδ2 T cells and type 1 natural killer T cells-in those with chronic lymphocytic leukemia, multiple myeloma, and acute myeloid leukemia.

The Fox Chase Cancer Center at Temple University in Philadelphia, Pennsylvania has been added as an active trial site by Salarius Pharmaceuticals, Inc. for the ongoing trial of seclidemstat for the treatment of relapsed or refractory Ewing sarcoma and advanced FET-rearranged sarcomas.

The investigational anti-TIGIT agent, COM902, has been administered to a patient for the first time as part of a phase 1 clinical trial evaluating COM902 in combination with the anti-PVRIG agent, COM701 in patients with advanced malignancies who have no alternative treatment options.

The first patient with a solid tumor has been dosed in the TiTAN-1 clinical trial, which is aiming to determine the safety, tolerability, and efficacy of GEN-011, a next-generation neoantigen adoptive cell therapy.

The first patient with an advanced solid tumor has been dosed in a phase 1 clinical trial of the multi-kinase inhibitor BTX-A51.

The investigational CDK4/6 ARK5 inhibitor, ON 123300, has been dosed in the first time in a patient whose is enrolled in a phase 1 trial of ON 123300 for the treatment of advanced cancers.

The first patient has been dosed in a study designed to evaluate the safety and feasibility of IL13Rα2 chimeric antigen receptor T cell therapy for the treatment of leptomeningeal brain tumors, such as glioblastoma, ependymoma, and medulloblastoma.

A phase 3 study with the goal of demonstrating overall survival benefit with imetelstat versus best available therapy in patients with refractory myelofibrosis has dosed the first patient with the experimental agent.

The novel combined immunotherapy CRX100 has been dosed in the first patient with refractory solid tumors in a phase 1, open-label, dose-escalation study.

The first patient with acute myeloid leukemia in a phase 2 trial of the MultiTAA-specific T cell, MT-401 followed stem-cell transplant has been dosed with the agent, according to a press release by Marker Therapeutics, Inc.

The first patient has been dosed in the phase 1/2 clinical trial of an oral CDK9 inhibitor targeting MYC-amplified solid tumors known as KB-0742.

In the phase 1 clinical trial of NBTXR3 with chemotherapy and radiation therapy as treatment of patients with esophageal cancer, the first patient has been dosed with the investigational tumor-agnostic radioenhancer.
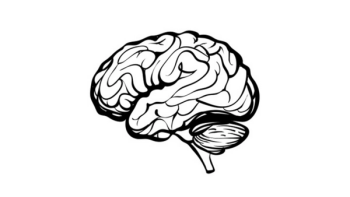
The first patient with newly diagnosed glioblastoma multiforme has been dosed with the first-in-class small molecule enzastaurin in combination with temozolomide and radiotherapy in the phase 3 ENGAGE clinical trial with an aim of determining the overall survival outcome of the drug in this patient population.

A phase 3 study was initiated for a head-to-head comparison of trastuzumab deruxtecan versus T-DM1 as adjuvant treatment of a subset of patients with HER2-positive early breast cancer.

Enzastaurin is on track to serve an unmet medical need in the treatment landscape of glioblastoma. Neuro-oncologists are waiting on the launch of an important clinical trial to further demonstrate the efficacy of the drug.

A pivotal phase 3 trial has been initiated to explore the combination of cabozantinib and atezolizumab for the treatment of patients with inoperable, locally advanced or metastatic renal cell carcinoma who progressed during or after treatment with an immune checkpoint inhibitor immediately following initial therapy.

The new phase III MOMENTUM clinical trial, which is evaluating the efficacy of momelotinib, a JAK1, JAK2 and ACVR1 inhibitor, versus active comparator danazol in patients with symptomatic and anemic myelofibrosis, was recently launched globally, according to a press release from Sierra Oncology.