
Bladder cancer is the fourth most prevalent cancer in the United States and is estimated to account for 7% of all new cancer cases in 2014.

Bladder cancer is the fourth most prevalent cancer in the United States and is estimated to account for 7% of all new cancer cases in 2014.

Based upon the stage of disease, which is indicative of invasiveness, different treatment regimens have been adopted and are included in guidelines developed by the NCCN.

Updated findings from the KEYNOTE-012 trial have demonstrated promising antitumor activity with pembrolizumab (Keytruda) in patients with metastatic gastric cancer.

Patients with hepatocellular carcinoma (HCC) that was related to hepatitis B virus (HBV) infection were found to have more advanced clinicopathologic features and worse outcomes compared with those with hepatitis C virus (HCV)-related HCC.
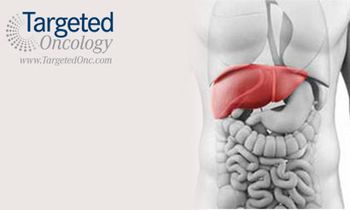
A collection of early phase clinical trials presented at the 2015 ASCO Annual Meeting demonstrated promising results for several novel agents in combination with established treatments for patients with hepatocellular carcinoma (HCC).

Rucaparib showed an overall response rate of 82% among a cohort of patients with BRCA-mutated ovarian cancer in the phase II ARIEL2 study.

The FDA has granted a priority review to pembrolizumab as a potential treatment for patients with advanced non-small cell lung cancer following treatment with chemotherapy or a targeted therapy, if applicable.
Immune checkpoint inhibition will continue to attract interest at the 2015 ASCO Annual Meeting, with a number of abstracts focused on this emerging class of agents

The House of Representatives Energy and Commerce Committee unanimously approved the 21st Century Cures Act in a 51-0 vote that took place last week.

Treatment with the PD-1 inhibitor nivolumab demonstrated similar efficacy regardless of prior treatment with a BRAF inhibitor or ipilimumab in patients with BRAF mutant or wild type metastatic melanoma.

Optimal outcomes, and perhaps even a cure, could be achieved with the combination of targeted therapies and immune checkpoint inhibitors.

TAS-102 (tipiracil hydrochloride) improved overall survival (OS) compared with placebo for patients with refractory metastatic colorectal cancer (CRC).

Treatment with the BTK inhibitor ibrutinib could enhance the efficacy of chemoimmunotherapy without increasing toxicity for patients with chronic lymphocytic leukemia.

Treatment with lapatinib plus a taxane was associated with a shorter duration of progression-free survival compared with trastuzumab plus a taxane as a frontline therapy for patients with HER2-positive metastatic breast cancer.

The level of TILs identified at the time of diagnosis were found to be an independent prognostic marker for pathologic complete response and event-free survival in patients treated with neoadjuvant HER2-target therapy plus chemotherapy for HER2-positive early breast cancer.

The added overall survival benefit seen with intraperitoneal versus intravenous chemotherapy extends beyond 10 years for patients with ovarian cancer.

A wide-ranging analysis of more than 5500 breast cancer tumors that combined genomic and protein expression testing has identified promising targets to explore for treating patients with poor prognoses, with particularly notable findings involving androgen receptor (AR) expression.
The investigational agent lenvatinib (E7808) met its primary endpoint of progression-free survival (PFS) in the phase III SELECT trial, which compared lenvatinib to placebo in patients with radioiodine-refractory differentiated thyroid cancer (RR-DTC),according to Eisai Inc., the company that is developing the agent.

Palbociclib plus letrozole is tolerable and more than doubles progression-free survival (PFS) for postmenopausal patients with locally advanced or newly diagnosed estrogen receptor (ER)-positive, HER2-negative metastatic breast cancer, according to final results from the phase II PALOMA-1 trial

Patients with bone metastatic castration-resistant prostate cancer (CRPC) who received radium-223 dichloride (Xofigo) continued to have a low incidence of myelosuppression and no associated secondary malignancies at a 1.5-year follow-up of the pivotal phase III ALSYMPCA study that was presented at the 2014 Genitourinary Cancers Symposium.
AGS-003, an investigational autologous dendritic cell vaccine, successfully activated a cytotoxic T cell response that correlates with a prolongation in survival for patients with metastatic renal cell carcinoma (mRCC), according to an analysis presented at the 2013 Annual Meeting of the Society for Immunotherapy of Cancer.

Across several studies, the BRAFV600E mutation has been reported to be associated with several negative prognostic clinicopathologic features as well as an increase in overall mortality in patients with papillary thyroid carcinoma (PTC).
The FDA has granted a Breakthrough Therapy designation to the novel PLK1 inhibitor volasertib in combination with LDAC for its potential as a treatment for patients with untreated AML who are ineligible for intensive remission induction therapy.
The FDA has granted Priority Review designation to dabrafenib (Tafinlar) and trametinib (Mekinist) as a combination treatment for patients with unresectable or metastatic melanoma with a BRAFV600E/K mutation.

The FDA’s Oncologic Drugs Advisory Committee voted 13-0 with one abstention in support of pertuzumab (Perjeta) in combination with trastuzumab and docetaxel for patients with HER2-positive breast cancer in the neoadjuvant setting.

Entinostat in combination with exemestane has been granted a Breakthrough Therapy designation from the FDA for its potential to reverse resistance to hormonal therapies used to treat patients with advanced ER-positive breast cancer.
Nab-paclitaxel (Abraxane) plus gemcitabine received FDA approval today as a treatment for patients with metastatic adenocarcinoma of the pancreas
An investigational MAGE-A3 vaccine failed to significantly extend disease-free survival (DFS) in certain patients with postsurgical melanoma

A phase III clinical program to investigate olaparib as a treatment for patients with BRCA-mutated ovarian cancer marks a step forward in the revived development of an agent that was once left on the sidelines.
Sorafenib (Nexavar) has been granted a priority review designation by the FDA for locally advanced or metastatic radioactive iodine (RAI)-refractory differentiated thyroid cancer.