An impressive array of newly approved treatments, as well as investigational agents, for non–small cell lung cancer (NSCLC) emerged in the first 6 months of 2015.

An impressive array of newly approved treatments, as well as investigational agents, for non–small cell lung cancer (NSCLC) emerged in the first 6 months of 2015.

One gene can encode more than one protein; however, proteins are dynamic (binding to membranes, other proteins, synthesis, degradation), undergo co- and post-translational modification, and exist in a wide range of concentrations in the body.

Researchers from Saint Louis University have developed a new class of anticancer drugs that potently reduce tumor growth and survival by targeting two energy sources, the Warburg effect (glycolysis) and lipogenesis.

At the ASCO 2015 Annual Meeting, F. Stephen Hodi, MD presented an analysis of the phase II CheckMate 069 trial covering objective response rate, progression-free survival, and safety in predefined subgroups, including those with poor prognostic factors.

At the 2015 ASCO Annual Meeting, Adil I. Daud, MD, UCSF Hellen Diller Family Comprehensive Cancer Center, presented a pooled analysis of 655 patients with advanced melanoma enrolled in the KEYNOTE-001 trial.
Researchers at the Wistar Institute Cancer Center have discovered a new potential circulating biomarker for non–small cell lung cancer. It is a cancer testis antigen expressed by a cancer/testis gene called AKAP4. The exciting prospect this heralds is the development of an accurate, quick blood test for early-stage NSCLC.

A team at Weill Cornell Medical College in New York that is studying ovarian cancer has not only discovered another mechanism by which tumors evade attack by the immune system, but is also devising a first-in-class potential treatment.

The vortex fluidic device, invented by Colin Raston FRACI CChem FRSC, is capable of “uncooking†an egg, but latest research demonstrates it can also manufacture new targeted cancer therapies at lower costs.

Results from the phase II, double-blind CheckMate-069 clinical trial showed unprecedented response rates with ipilimumab combined with nivolumab in previously treated patients with metastatic melanoma.
A delay between the diagnosis of melanoma and needed surgery may evoke anxiety and psychological stress; although the impact on morbidity and mortality remains controversial.
Turning their experimental focus on the tumor microenvironment, the authors of a paper published in Clinical Cancer Research have shed light on the role of melanoma-associated tumor macrophages in resistance to BRAF V600E inhibitors.

Immunotherapy is a rapidly expanding approach to the treatment of melanoma, employing a number of strategies evident in the pipeline for immunotherapeutics.

Only about 5% of patients with cancer are recruited into oncology clinical trials, raising a concern that unless this improves, progress in the development new treatments for cancer may be delayed.

A key to improved outcomes for patients with non-small cell lung cancer (NSCLC) is the ability to identify the wide spectrum of genetic changes present in tumors. Doing so will guide treatment decisions and influence the development of much-needed additional targeted therapies.

The Cancer Genome Atlas (TCGA) began in 2006, with a total investment of $100 million from the National Cancer Institute and the National Human Genome Research Institute for a 3-year pilot period.
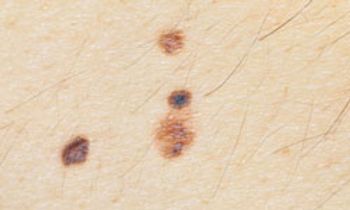
Only in the last 2 decades or so have sufficient data become available to statistically associate high mitotic rate with survival in melanomas.