Articles by Barbara L. Jones

More than two dozen abstracts at the December 2013 meeting of the American Society of Hematology (ASH) focused on the detection, measurement, or monitoring of MRD in patients with multiple myeloma.
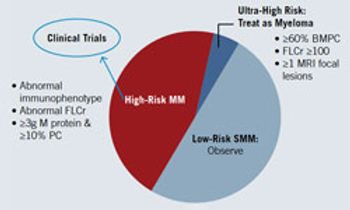
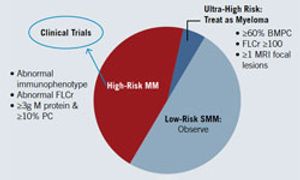
In most malignancies, early detection and intervention are prerequisites for a possible cure. Consequently, why is the standard of care for myeloma a watch-and-wait approach, with no treatment until progression?
Although the current standard of care for advanced NSCLC remains platinum doublet chemotherapy, recent evidence suggests that most newly diagnosed patients may be candidates for targeted therapy as firstline treatment.

Between 2007 and 2011, a collaboration among clinical oncologists, pathologists, and industry scientists led to the identification of a new molecularly defined subset of NSCLC, followed by the finding that crizotinib, then under development as a MET inhibitor, was an inhibitor of ALK.

T-VEC, a novel oncolytic immunotherapy derived from the herpes simplex virus type 1, demonstrated a significant improvement in DRR, the primary endpoint in a pivotal phase III trial in patients with stage IIIB-IV melanoma.

MPDL3280A produced durable responses in studies in patients with forms of locally advanced/metastatic cancers, including smokers with NSCLC who customarily have poorer responses to cancer therapies than nonsmokers.

Targeted Therapies spoke with Suzanne Topalian, MD, professor of Surgery and Oncology at Johns Hopkins University School of Medicine, about the progress and promise of PD-1 targeted cancer therapy.

PD-L1 expression in tumors is a candidate molecular marker warranting further investigation as a means to select patients for immunotherapy with an anti PD-1 antibody

Concurrent nivolumab and ipilimumab produced “rapid and deep†responses in patients with advanced melanoma who took part in the first phase I trial to evaluate the PD-1-blocking antibody nivolumab, along with the CTLA-4-blocking antibody ipilimumab.
It was hoped that novel anticancer therapies, by directly targeting aberrant cancer cells, would avoid the toxicities of traditional cytotoxic agents. Instead, the range of expected toxicities has widened.

In an updated analysis of the phase III MM-003 trial, Celgene International Sarl, the makers of pomalidomide, reported on a new progression-free survival (PFS) analysis and final overall survival (OS) in September 2013.

With the arrival and incorporation into clinical practice of immunomodulatory drugs and proteasome inhibitor therapy, patients with multiple myeloma patients are achieving deep, durable responses and disease control, and are living longer

Oral proteasome inhibitor to be studied in phase III trials, identifying new targets, pomalidomide for relapsed/refractory MM, early stage data for daratumumab, and updated phase I and II results for elotuzumab in multiple myeloma.

Three presentations from the ASCO 2013 Annual Meeting extended positive findings comparing nab-paclitaxel and gemcitabine to gemcitabine in 861 untreated patients with advanced pancreatic cancer.